The Gcn5-related N-acetyltransferase Superfamily
Why Are We Studying GNATs?
The large Gcn5-related N-acetyltransferase (GNAT) superfamily is comprised of over 200,000 members from more than 20,000 species in all kingdoms of life. GNATs catalyze the transfer of an acyl-group from a donor molecule to an acceptor molecule. Typically, acetyl coenzyme A (AcCoA) acts as the donor, and acyl-group transfer is usually to a primary amino group of an acceptor molecule. The functional diversity of members of this family of proteins is vast, with important roles in metabolic and cellular processes, gene regulation, transcription, detoxification, drug resistance, and post-translational protein modification. Only small subset of GNATs of all kingdoms of life has been studied, so the functions of the majority of these proteins remain unknown. Our NSF-funded investigations into functions of uncharacterized GNATs are providing new insight into currently unknown biochemical and cellular processes through the design and synthesis of new tools to structurally and functionally characterize GNATs of unknown function.

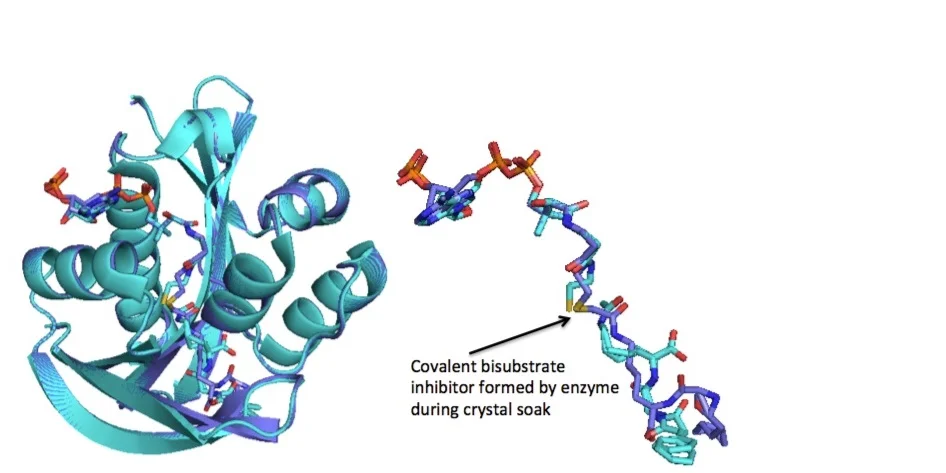
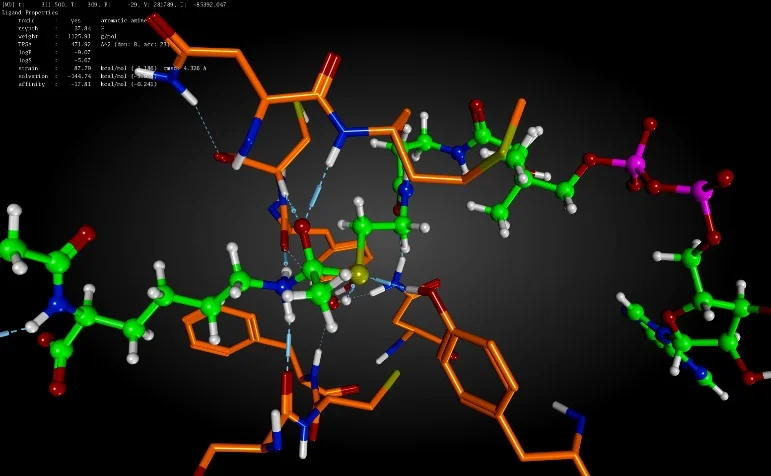
Enzyme-catalyzed reaction of modified substrate and CoA to form covalent bisubstrate and crystal structure of PA4794 in ternary complex (PDB ID: 4L8A; cyan) and in presence of covalent bisubstrate (purple). Formation of the covalent bisubstrate was enzyme-catalyzed during crystal soak.